Human Biomonitoring Guidance Values (HBM-GVs) for Bisphenol S and Assessment of the Risk Due to the Exposure to Bisphenols A and S, in Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation of Risk
2.2. Derivation of HBM-GVs: General Methodology
2.3. Characterisation of Internal Exposure to BPA and BPS
3. Results
3.1. Assessment of Risk Due to BPA Exposure
3.1.1. Derivation of HBM-GVs for BPA
Derivation of HBM-GVGenPop
Derivation of HBM-GVworker
3.1.2. Assessment of Exposure to BPA
3.1.3. Risk Characterisation Due to BPA Exposure
For the General Population
| Cohorts | References | Country | Populations | P95 (µg/L) | HBM-GVGenPop for Total Urinary BPA (µg/L) | RCR |
|---|---|---|---|---|---|---|
| IBS | Berman et al., 2014 [40] | Israel | Adults (40–59 years old) | 20.48 | 230 | 0.09 |
| GerES IV * | Becker et al., 2009 [41] | Germany | Children (3–14 years old) | 14 | 135 | 0.10 |
| GerES V | Tschersich et al., 2021 [42] | Germany | Children (3–5 years old) | 7.79 | 135 | 0.06 |
| Children (6–10 years old) | 5.13 | 135 | 0.04 | |||
| Children (11–13 years old) | 9.95 | 135 | 0.07 | |||
| Adolescents (14–17 years old) | 7.65 | 230 | 0.03 | |||
| 3xG | Study website [43] | Belgium | Adults (20–39 years old) | 4.61 | 230 | 0.02 |
| FLEHS II | Geens et al., 2014 [44] | Belgium | Adolescents (14–15 years old) | 9.6 | 230 | 0.04 |
| DEMOCOPHES | Covaci et al., 2015 [45] | Belgium, Denmark, Luxembourg, Slovenia, Spain, Sweden | Mothers (<45 years old) | 11.1 | 230 | 0.05 |
| Children (5–12 years old) | 13.1 | 135 | 0.10 | |||
| Belgium | Mothers (<45 years old) | 11.6 | 230 | 0.05 | ||
| Children (5–12 years old) | 13.4 | 135 | 0.10 | |||
| Denmark | Mothers (<45 years old) | 11.5 | 230 | 0.05 | ||
| Children (5–12 years old) | 7.9 | 135 | 0.06 | |||
| Luxembourg | Mothers (<45 years old) | 7.4 | 230 | 0.03 | ||
| Children (5–12 years old) | 8.3 | 135 | 0.06 | |||
| Slovenia | Mothers (<45 years old) | 13.4 | 230 | 0.06 | ||
| Children (5–12 years old) | 18.9 | 135 | 0.14 | |||
| Spain | Mothers (<45 years old) | 12.2 | 230 | 0.05 | ||
| Children (5–12 years old) | 9.8 | 135 | 0.07 | |||
| Sweden | Mothers (<45 years old) | 5 | 230 | 0.02 | ||
| Children (5–12 years old) | 6.2 | 135 | 0.05 | |||
| PBAT | Hartmann et al., 2016 [46] | Austria | Children M (6–10 years old) | 7.3 | 135 | 0.05 |
| Children F (6–10 years old) | 5.8 | 135 | 0.04 | |||
| Teenagers M (11–15 years old) | 2.7 | 230 | 0.01 | |||
| Teenagers F (11–15 years old) | 5.2 | 230 | 0.02 | |||
| Adults M (18–64 years old) | 3.6 | 230 | 0.02 | |||
| Adults F (18–64 years old) | 1.3 | 230 | 0.01 | |||
| Senior M (65–79 years old) | 1.3 | 230 | 0.01 | |||
| Senior M (65–79 years old) | 4.6 | 230 | 0.02 | |||
| HELIX ** | Haug et al., 2018 [47] | France, Greece, Lithuania, Norway, Spain, United Kingdom. | Pregnant women (>18 years old) | 22 | 230 | 0.10 |
| Children (6–12 years old) | 15.7 | 135 | 0.12 | |||
| Elfe | Dereumeaux et al., 2016 [48] | France | Pregnant women (>18 years old) | 5.3 | 230 | 0.02 |
| Esteban | Balicco et al., 2019 [49] | France | Children (6–10 years old) | 7.3 | 135 | 0.05 |
| Adolescents (11–14 years old) | 13.7 | 230 | 0.06 | |||
| Adolescents (15–17 years old) | 6 | 230 | 0.03 | |||
| Adults M (18–74 years old) | 10 | 230 | 0.04 | |||
| Adults F (18–74 years old) | 6.9 | 230 | 0.03 | |||
| Danish-HBM | Frederiksen et al., 2014 [50] | Denmark | Pregnant women (>18 years old) | 7.52 | 230 | 0.03 |
| RefLim 2011 | Porras et al., 2014 [51] | Finland | Adults (22–67 years old) | 7.9 | 230 | 0.03 |
| RHEA | Myridakis et al., 2015 [52] | Greece | Pregnant women (>16 years old) | 4.7 | 230 | 0.02 |
| Children (4 years old) | 16.6 | 135 | 0.12 | |||
| INMA | Casas et al., 2013 [53] | Spain | Pregnant women (≥16 years) first trimester | 11.9 | 230 | 0.05 |
| Children (4 years old) | 12.3 | 135 | 0.09 | |||
| TH Pregnant women | Machtinger et al., 2018 [54] | Israel | Pregnant women | 14.2 | 230 | 0.06 |
| SLO-CRP | Tkalec et al., 2021 [55] | Slovenia | Children (6–9 years old) | 9.5 | 135 | 0.07 |
| Adolescents (11–15 years old) | 7.3 | 230 | 0.03 |
For Workers
3.2. Assessment of Risk Due to BPS Exposure
3.2.1. Derivation of HBM-GVs for BPS
Selection of the Methodological Approach for the Derivation of HBM-GVs for BPS
Selection of the Biomarker of Exposure for BPS
Selection of Key Studies and Choice of POD
Prediction of the Total Urinary BPS Concentration with a 100% Oral Exposure Scenario
3.2.2. Assessment of Exposure to BPS
3.2.3. Risk Characterisation Due to Exposure to BPS
For the General Population
For Workers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ANSES. Analysis of the Most Appropriate Risk Management Option (RMOA) for Bisphenol A; ECHA: Helsinki, Finland, 2017.
- EFSA. Panel on Food Contact Materisals, Enzymes, Flavourings and Processing Aids (CEF) Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Geens, T.; Goeyens, L.; Covaci, A. Are Potential Sources for Human Exposure to Bisphenol-A Overlooked? Int. J. Hydrogen Environ. Health 2011, 214, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Welshons, W.V. Evidence That Bisphenol A (BPA) Can Be Accurately Measured without Contamination in Human Serum and Urine, and That BPA Causes Numerous Hazards from Multiple Routes of Exposure. Mol. Cell. Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health Risk of Exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar]
- Bernier, M.R.; Vandenberg, L.N. Handling of Thermal Paper: Implications for Dermal Exposure to Bisphenol A and Its Alternatives. PLoS ONE 2017, 12, e0178449. [Google Scholar] [CrossRef]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef]
- ANSES. Évaluation Des Risques Du Bisphénol A (BPA) Pour La Santé Humaine. Available online: https://www.anses.fr/fr/system/files/CHIM2009sa0331Ra-0.pdf?msclkid=3c3a0147c61311eca4e97b466caa524b (accessed on 18 March 2022).
- FAO; WHO. Toxicological and Health Aspects of Bisphenol A; WHO: Ottawa, ON, Canada, 2011. [Google Scholar]
- US Food and Drug Administration Bisphenol A (BPA): Use in Food Contact Application. Available online: https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application (accessed on 18 March 2022).
- Kommission Human-Biomonitoring des Umweltbundesamtes Stoffmonographie Bisphenol A (BPA)—Referenz- und Human-Biomonitoring-(HBM)-Werte für BPA im Urin. Bundesgesundheitsblatt—Gesundh.—Gesundh. 2012, 55, 1215–1231. [CrossRef]
- Apel, P.; Angerer, J.; Wilhelm, M.; Kolossa-Gehring, M. New HBM Values for Emerging Substances, Inventory of Reference and HBM Values in Force, and Working Principles of the German Human Biomonitoring Commission. Int. J. Hydrogen Environ. Health 2017, 220, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Sloan, C.S.; Castillo, N.P.; Veselica, M.M.; Seely, J.C.; Dimond, S.S.; Van Miller, J.P.; Shiotsuka, R.N.; et al. Two-Generation Reproductive Toxicity Study of Dietary Bisphenol A in CD-1 (Swiss) Mice. Toxicol. Sci. 2008, 104, 362–384. [Google Scholar] [CrossRef]
- ECHA; Committee for Risk Assessment (RAC). Opinion on an Annex XV Dossier Proposing Restrictions on Bisphenol A. Available online: https://echa.europa.eu/documents/10162/209030fc-ca4b-4745-97b6-98bfc4d6bdd3 (accessed on 18 March 2022).
- Commission Regulation (EU) 2016/2235 of 12 December 2016 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bisphenol A (Text with EEA Relevance); Official Journal of the European Union, 2016; Volume 337.
- Danish, E.P.A. Alternative Technologies and Substances to Bisphenol A (BPA) in Thermal Paper Receipts; Danish Environmental Protection Agencey: Copenhagen, Denmark, 2014. [Google Scholar]
- ECHA. Bisphenol A. Available online: https://echa.europa.eu/hot-topics/bisphenol-a (accessed on 18 March 2022).
- EFSA. Panel on Food Contact Materials, Enzymes and Processing Aids Re-Evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs; EFSA: Parma, Italy, 2021.
- Česen, M.; Lenarčič, K.; Mislej, V.; Levstek, M.; Kovačič, A.; Cimrmančič, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S.; et al. The Occurrence and Source Identification of Bisphenol Compounds in Wastewaters. Sci. Total Environ. 2018, 616–617, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Campillo, N.; Martínez-Castillo, N.; Hernández-Córdoba, M. Comparison of Two Derivatization-Based Methods for Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometric Determination of Bisphenol A, Bisphenol S and Biphenol Migrated from Food Cans. Anal. Bioanal. Chem. 2010, 397, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- ECHA. Use of Bisphenol A and Its Alternatives in Thermal Paper in the EU 2014-16; ECHA: Helsinki, Finland, 2017.
- Yang, Y.; Yang, Y.; Zhang, J.; Shao, B.; Yin, J. Assessment of Bisphenol A Alternatives in Paper Products from the Chinese Market and Their Dermal Exposure in the General Population. Environ. Pollut. 2019, 244, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, C.; Le Magueresse-Battistoni, B.; Viguié, C.; Babajko, S.; Canivenc-Lavier, M.-C.; Chevalier, N.; Emond, C.; Habert, R.; Picard-Hagen, N.; Mhaouty-Kodja, S. Regulatory and Academic Studies to Derive Reference Values for Human Health: The Case of Bisphenol S. Environ. Res. 2022, 204, 112233. [Google Scholar] [CrossRef]
- Ganzleben, C.; Antignac, J.-P.; Barouki, R.; Castaño, A.; Fiddicke, U.; Klánová, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human Biomonitoring as a Tool to Support Chemicals Regulation in the European Union. Int. J. Hydrogen Environ. Health 2017, 220, 94–97. [Google Scholar] [CrossRef]
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human Biomonitoring in Health Risk Assessment in Europe: Current Practices and Recommendations for the Future. Int. J. Hydrogen Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.-P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human Biomonitoring Initiative (HBM4EU): Human Biomonitoring Guidance Values (HBM-GVs) Derived for Bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Apel, P.; Rousselle, C.; Lange, R.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. Human Biomonitoring Initiative (HBM4EU)—Strategy to Derive Human Biomonitoring Guidance Values (HBM-GVs) for Health Risk Assessment. Int. J. Hydrogen Environ. Health 2020, 230, 113622. [Google Scholar] [CrossRef]
- IPCheM Portal. Available online: https://ipchem.jrc.ec.europa.eu/ (accessed on 18 March 2022).
- Bousoumah, R.; Leso, V.; Iavicoli, I.; Huuskonen, P.; Viegas, S.; Porras, S.P.; Santonen, T.; Frery, N.; Robert, A.; Ndaw, S. Biomonitoring of Occupational Exposure to Bisphenol A, Bisphenol S and Bisphenol F: A Systematic Review. Sci. Total Environ. 2021, 783, 146905. [Google Scholar] [CrossRef]
- Karrer, C.; Roiss, T.; von Goetz, N.; Gramec Skledar, D.; Peterlin Mašič, L.; Hungerbühler, K. Physiologically Based Pharmacokinetic (PBPK) Modeling of the Bisphenols BPA, BPS, BPF, and BPAF with New Experimental Metabolic Parameters: Comparing the Pharmacokinetic Behavior of BPA with Its Substitutes. Environ. Health Perspect. 2018, 126, 077002. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Arbuckle, T.E.; Fisher, M. Adjusting Urinary Chemical Biomarkers for Hydration Status during Pregnancy. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-H.E.; Siskos, A.P.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T.; et al. Determinants of the Urinary and Serum Metabolome in Children from Six European Populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinälä, M.; Ylinen, K.; Tuomi, T.; Santonen, T.; Porras, S.P. Assessment of Occupational Exposure to Bisphenol A in Five Different Production Companies in Finland. Ann. Work Expo. Health 2017, 61, 44–55. [Google Scholar] [CrossRef]
- Simonelli, A.; Guadagni, R.; De Franciscis, P.; Colacurci, N.; Pieri, M.; Basilicata, P.; Pedata, P.; Lamberti, M.; Sannolo, N.; Miraglia, N. Environmental and Occupational Exposure to Bisphenol A and Endometriosis: Urinary and Peritoneal Fluid Concentration Levels. Int. Arch. Occup. Environ. Health 2017, 90, 49–61. [Google Scholar] [CrossRef]
- Lyapina, M.; Dencheva, M.S.; Krasteva, A.Z.; Nikolov, G.; Cekova, M.P.; Deliverska, M.; Kisselova-Yaneva, A. Biomonitoring of Urinary Levels of Bisphenol A. C. R. De L’académie Bulg. Des Sci. 2016, 69, 807–813. [Google Scholar]
- González, N.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Marquès, M.; Domingo, J.L.; Nadal, M. Quantification of Eight Bisphenol Analogues in Blood and Urine Samples of Workers in a Hazardous Waste Incinerator. Environ. Res. 2019, 176, 108576. [Google Scholar] [CrossRef]
- Ndaw, S.; Remy, A.; Jargot, D.; Robert, A. Occupational Exposure of Cashiers to Bisphenol A via Thermal Paper: Urinary Biomonitoring Study. Int. Arch. Occup. Environ. Health 2016, 89, 935–946. [Google Scholar] [CrossRef]
- Berman, T.; Goldsmith, R.; Göen, T.; Spungen, J.; Novack, L.; Levine, H.; Amitai, Y.; Shohat, T.; Grotto, I. Demographic and Dietary Predictors of Urinary Bisphenol A Concentrations in Adults in Israel. Int. J. Hydrogen Environ. Health 2014, 217, 638–644. [Google Scholar] [CrossRef]
- Becker, K.; Güen, T.; Seiwert, M.; Conrad, A.; Pick-Fuß, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate Metabolites and Bisphenol A in Urine of German Children. Int. J. Hydrogen Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef]
- Tschersich, C.; Murawski, A.; Schwedler, G.; Rucic, E.; Moos, R.K.; Kasper-Sonnenberg, M.; Koch, H.M.; Brüning, T.; Kolossa-Gehring, M. Bisphenol A and Six Other Environmental Phenols in Urine of Children and Adolescents in Germany—Human Biomonitoring Results of the German Environmental Survey 2014–2017 (GerES V). Sci. Total Environ. 2021, 763, 144615. [Google Scholar] [CrossRef] [PubMed]
- 3xg Studie|3xgstudie. Available online: https://studie3xg.be/nl (accessed on 18 March 2022).
- Geens, T.; Bruckers, L.; Covaci, A.; Schoeters, G.; Fierens, T.; Sioen, I.; Vanermen, G.; Baeyens, W.; Morrens, B.; Loots, I.; et al. Determinants of Bisphenol A and Phthalate Metabolites in Urine of Flemish Adolescents. Environ. Res. 2014, 134, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Hond, E.D.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Mørck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA Measurements in Children and Mothers from Six European Member States: Overall Results and Determinants of Exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Uhl, M.; Weiss, S.; Scharf, S.; König, J. Human Biomonitoring of Bisphenol A Exposure in an Austrian Population. Biomonitoring 2016, 3, 5–14. [Google Scholar] [CrossRef]
- Haug, L.S.; Sakhi, A.K.; Cequier, E.; Casas, M.; Maitre, L.; Basagana, X.; Andrusaityte, S.; Chalkiadaki, G.; Chatzi, L.; Coen, M.; et al. In-Utero and Childhood Chemical Exposome in Six European Mother-Child Cohorts. Environ. Int. 2018, 121, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; de Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; le Tertre, A.; Lefranc, A.; et al. Biomarkers of Exposure to Environmental Contaminants in French Pregnant Women from the Elfe Cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef]
- Balicco, A.; Bidondo, M.; Fillol, C.; Gane, J.; Oleko, A.; Saoudi, A.; Zeghnoun, A. Imprégnation de la population française par les bisphénols A, S et F: Programme national de biosurveillance, Esteban 2014–2016; Santé Publique France: Saint-Maurice, France, 2019. [Google Scholar]
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A.-M. Human Urinary Excretion of Non-Persistent Environmental Chemicals: An Overview of Danish Data Collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef]
- Porras, S.P.; Heinälä, M.; Santonen, T. Bisphenol A Exposure via Thermal Paper Receipts. Toxicol. Lett. 2014, 230, 413–420. [Google Scholar] [CrossRef]
- Myridakis, A.; Fthenou, E.; Balaska, E.; Vakinti, M.; Kogevinas, M.; Stephanou, E.G. Phthalate Esters, Parabens and Bisphenol-A Exposure among Mothers and Their Children in Greece (Rhea Cohort). Environ. Int. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Casas, M.; Valvi, D.; Luque, N.; Ballesteros-Gomez, A.; Carsin, A.-E.; Fernandez, M.F.; Koch, H.M.; Mendez, M.A.; Sunyer, J.; Rubio, S.; et al. Dietary and Sociodemographic Determinants of Bisphenol A Urine Concentrations in Pregnant Women and Children. Environ. Int. 2013, 56, 10–18. [Google Scholar] [CrossRef]
- Machtinger, R.; Berman, T.; Adir, M.; Mansur, A.; Baccarelli, A.A.; Racowsky, C.; Calafat, A.M.; Hauser, R.; Nahum, R. Urinary Concentrations of Phthalate Metabolites, Bisphenols and Personal Care Product Chemical Biomarkers in Pregnant Women in Israel. Environ. Int. 2018, 116, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, Ž.; Kosjek, T.; Snoj Tratnik, J.; Stajnko, A.; Runkel, A.A.; Sykiotou, M.; Mazej, D.; Horvat, M. Exposure of Slovenian Children and Adolescents to Bisphenols, Parabens and Triclosan: Urinary Levels, Exposure Patterns, Determinants of Exposure and Susceptibility. Environ. Int. 2021, 146, 106172. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Doerge, D.R.; Teeguarden, J.G.; Fisher, J.W. Development of a Physiologically Based Pharmacokinetic Model for Assessment of Human Exposure to Bisphenol A. Toxicol. Appl. Pharmacol. 2015, 289, 442–456. [Google Scholar] [CrossRef]
- Oh, J.; Choi, J.W.; Ahn, Y.-A.; Kim, S. Pharmacokinetics of Bisphenol S in Humans after Single Oral Administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Choi, J.W.; Ahn, Y.-A.; Kim, S. Response to the Letter to the Editor. Environ. Int. 2018, 115, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Khmiri, I.; Côté, J.; Mantha, M.; Khemiri, R.; Lacroix, M.; Gely, C.; Toutain, P.-L.; Picard-Hagen, N.; Gayrard, V.; Bouchard, M. Toxicokinetics of Bisphenol-S and Its Glucuronide in Plasma and Urine Following Oral and Dermal Exposure in Volunteers for the Interpretation of Biomonitoring Data. Environ. Int. 2020, 138, 105644. [Google Scholar] [CrossRef]
- Champmartin, C.; Marquet, F.; Chedik, L.; Décret, M.-J.; Aubertin, M.; Ferrari, E.; Grandclaude, M.-C.; Cosnier, F. Human in Vitro Percutaneous Absorption of Bisphenol S and Bisphenol A: A Comparative Study. Chemosphere 2020, 252, 126525. [Google Scholar] [CrossRef]
- Zalko, D.; Soto, A.M.; Dolo, L.; Dorio, C.; Rathahao, E.; Debrauwer, L.; Faure, R.; Cravedi, J.-P. Biotransformations of Bisphenol A in a Mammalian Model: Answers and New Questions Raised by Low-Dose Metabolic Fate Studies in Pregnant CD1 Mice. Environ. Health Perspect. 2003, 111, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Gramec Skledar, D.; Peterlin Mašič, L. Bisphenol A and Its Analogs: Do Their Metabolites Have Endocrine Activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef]
- Kolla, S.; Morcos, M.; Martin, B.; Vandenberg, L.N. Low Dose Bisphenol S or Ethinyl Estradiol Exposures during the Perinatal Period Alter Female Mouse Mammary Gland Development. Reprod. Toxicol. 2018, 78, 50–59. [Google Scholar] [CrossRef]
- Kolla, S.; McSweeney, D.B.; Pokharel, A.; Vandenberg, L.N. Bisphenol S Alters Development of the Male Mouse Mammary Gland and Sensitizes It to a Peripubertal Estrogen Challenge. Toxicology 2019, 424, 152234. [Google Scholar] [CrossRef] [PubMed]
- Catanese, M.C.; Vandenberg, L.N. Bisphenol S (BPS) Alters Maternal Behavior and Brain in Mice Exposed during Pregnancy/Lactation and Their Daughters. Endocrinology 2017, 158, 516–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r8_en.pdf/e153243a-03f0-44c5-8808-88af66223258?t=1353935239897 (accessed on 18 March 2022).
- Husøy, T.; Andreassen, M.; Hjertholm, H.; Carlsen, M.H.; Norberg, N.; Sprong, C.; Papadopoulou, E.; Sakhi, A.K.; Sabaredzovic, A.; Dirven, H.A.A.M. The Norwegian Biomonitoring Study from the EU Project EuroMix: Levels of Phenols and Phthalates in 24-Hour Urine Samples and Exposure Sources from Food and Personal Care Products. Environ. Int. 2019, 132, 105103. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Sabaredzovic, A.; Papadopoulou, E.; Cequier, E.; Thomsen, C. Levels, Variability and Determinants of Environmental Phenols in Pairs of Norwegian Mothers and Children. Environ. Int. 2018, 114, 242–251. [Google Scholar] [CrossRef]
- Ndaw, S.; Remy, A.; Denis, F.; Marsan, P.; Jargot, D.; Robert, A. Occupational Exposure of Cashiers to Bisphenol S via Thermal Paper. Toxicol. Lett. 2018, 298, 106–111. [Google Scholar] [CrossRef]
- Almeida, D.L.; Pavanello, A.; Saavedra, L.P.; Pereira, T.S.; de Castro-Prado, M.A.A.; de Mathias, P.C.F. Environmental Monitoring and the Developmental Origins of Health and Disease. J. Dev. Orig. Health Dis. 2019, 10, 608–615. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of Bisphenol A on Metabolism and Evidences of a Mode of Action Mediated through Endocrine Disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef]
- Mhaouty-Kodja, S.; Belzunces, L.P.; Canivenc, M.-C.; Schroeder, H.; Chevrier, C.; Pasquier, E. Impairment of Learning and Memory Performances Induced by BPA: Evidences from the Literature of a MoA Mediated through an ED. Mol. Cell. Endocrinol. 2018, 475, 54–73. [Google Scholar] [CrossRef]
- Christensen, K.L.Y.; Lorber, M.; Koch, H.M.; Kolossa-Gehring, M.; Morgan, M.K. Population Variability of Phthalate Metabolites and Bisphenol A Concentrations in Spot Urine Samples versus 24- or 48-h Collections. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 632–640. [Google Scholar] [CrossRef]
- Vernet, C.; Philippat, C.; Calafat, A.M.; Ye, X.; Lyon-Caen, S.; Siroux, V.; Schisterman, E.F.; Slama, R. Within-Day, Between-Day, and Between-Week Variability of Urinary Concentrations of Phenol Biomarkers in Pregnant Women. Environ. Health Perspect. 2018, 126, 037005. [Google Scholar] [CrossRef]

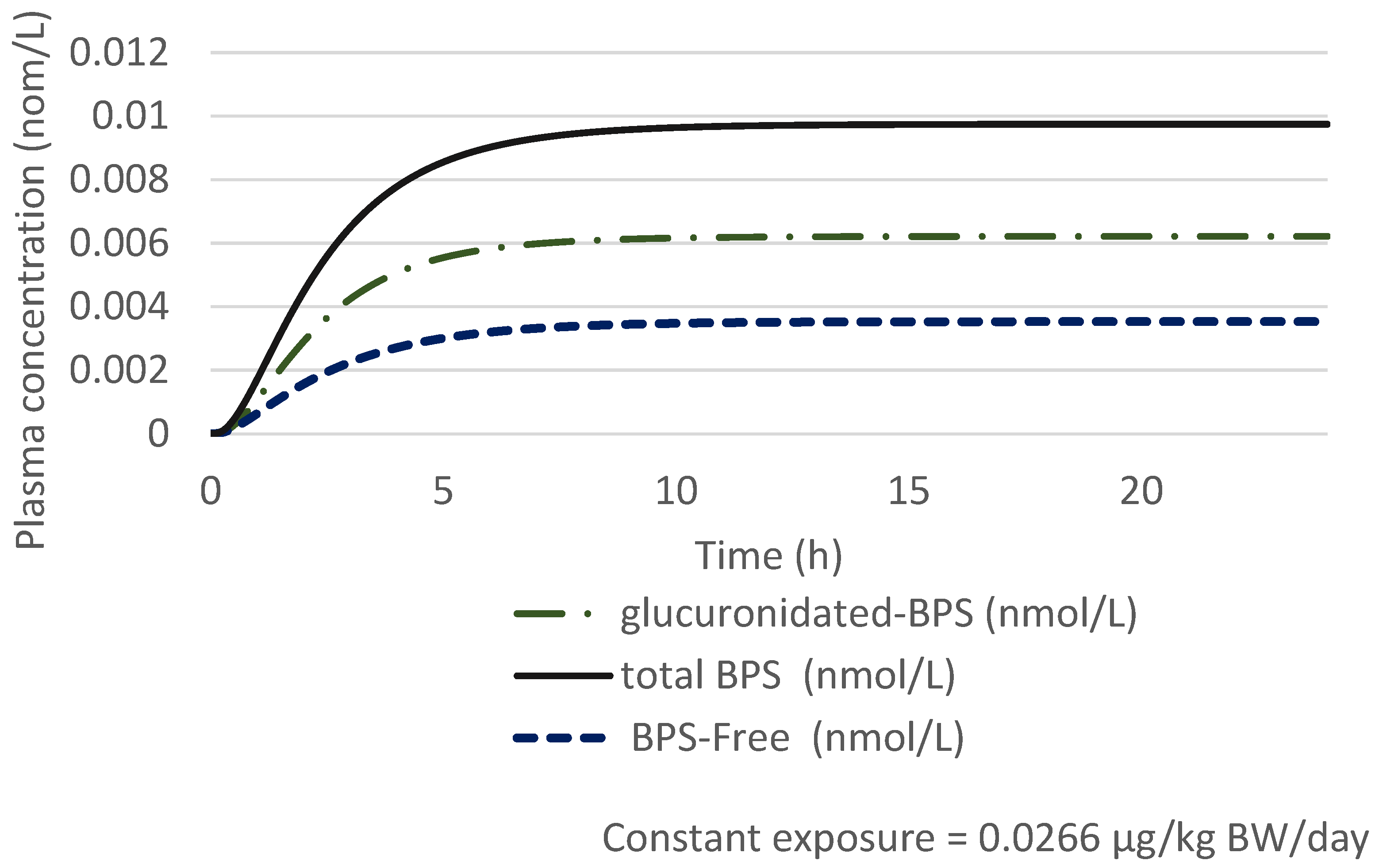
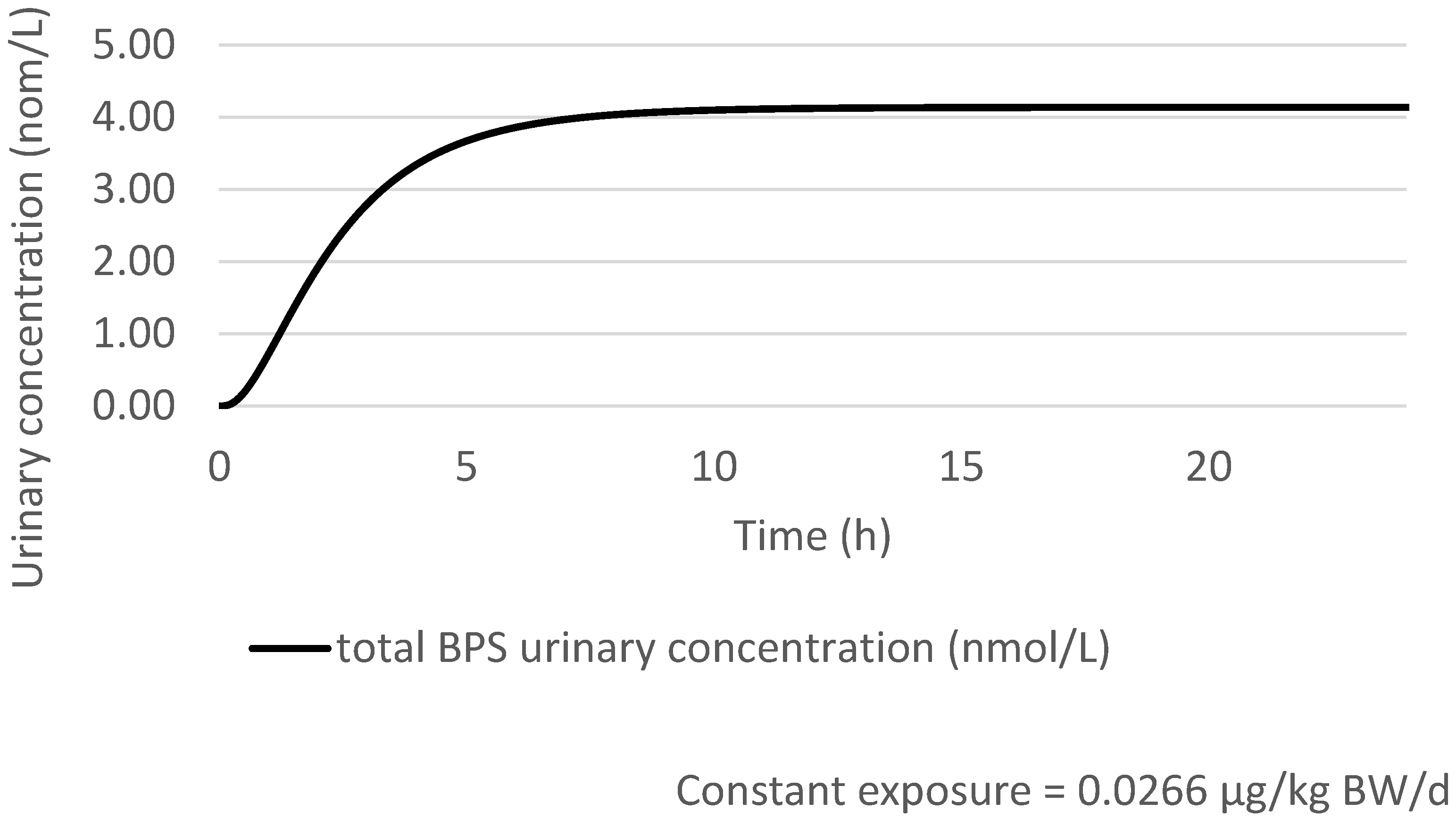
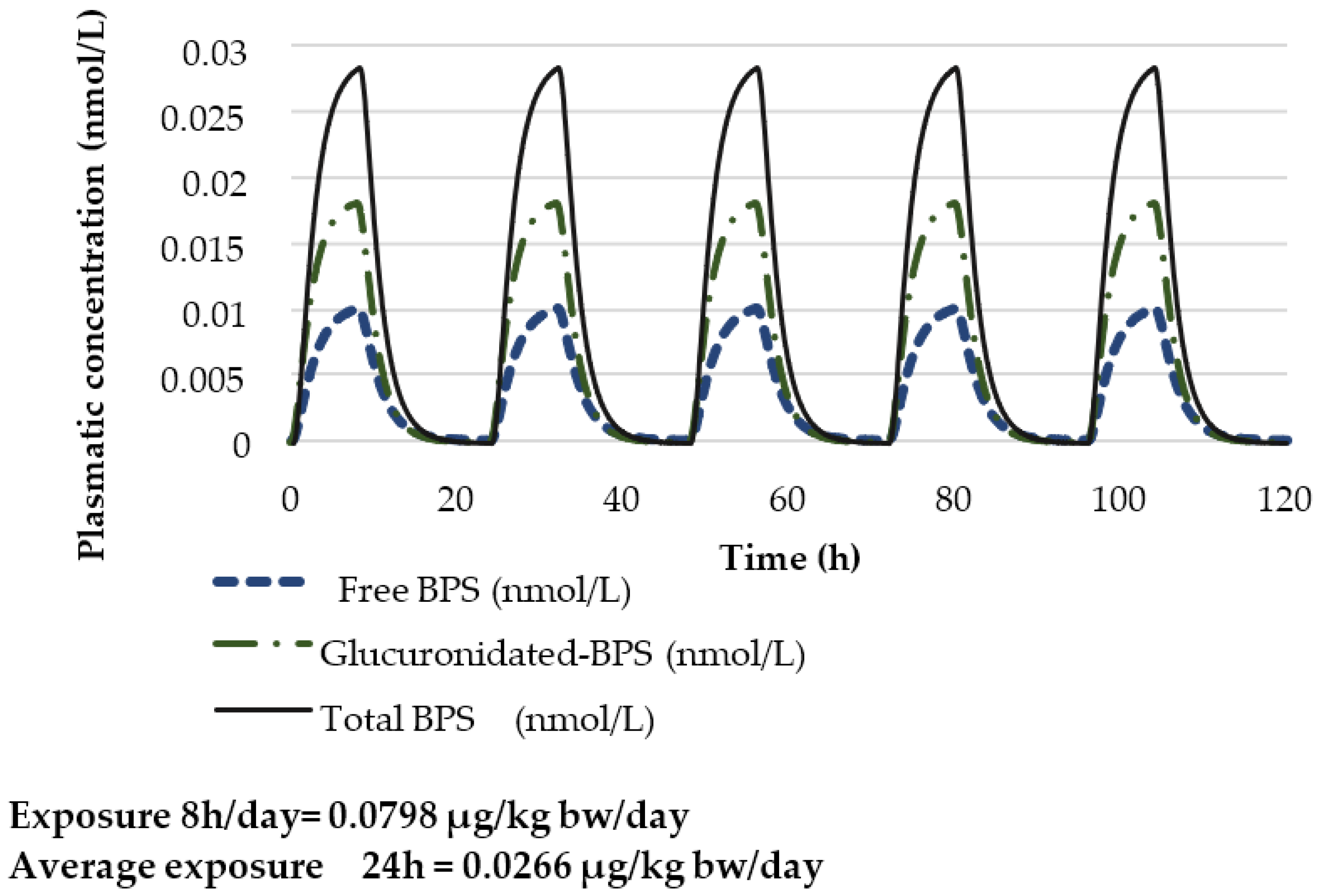
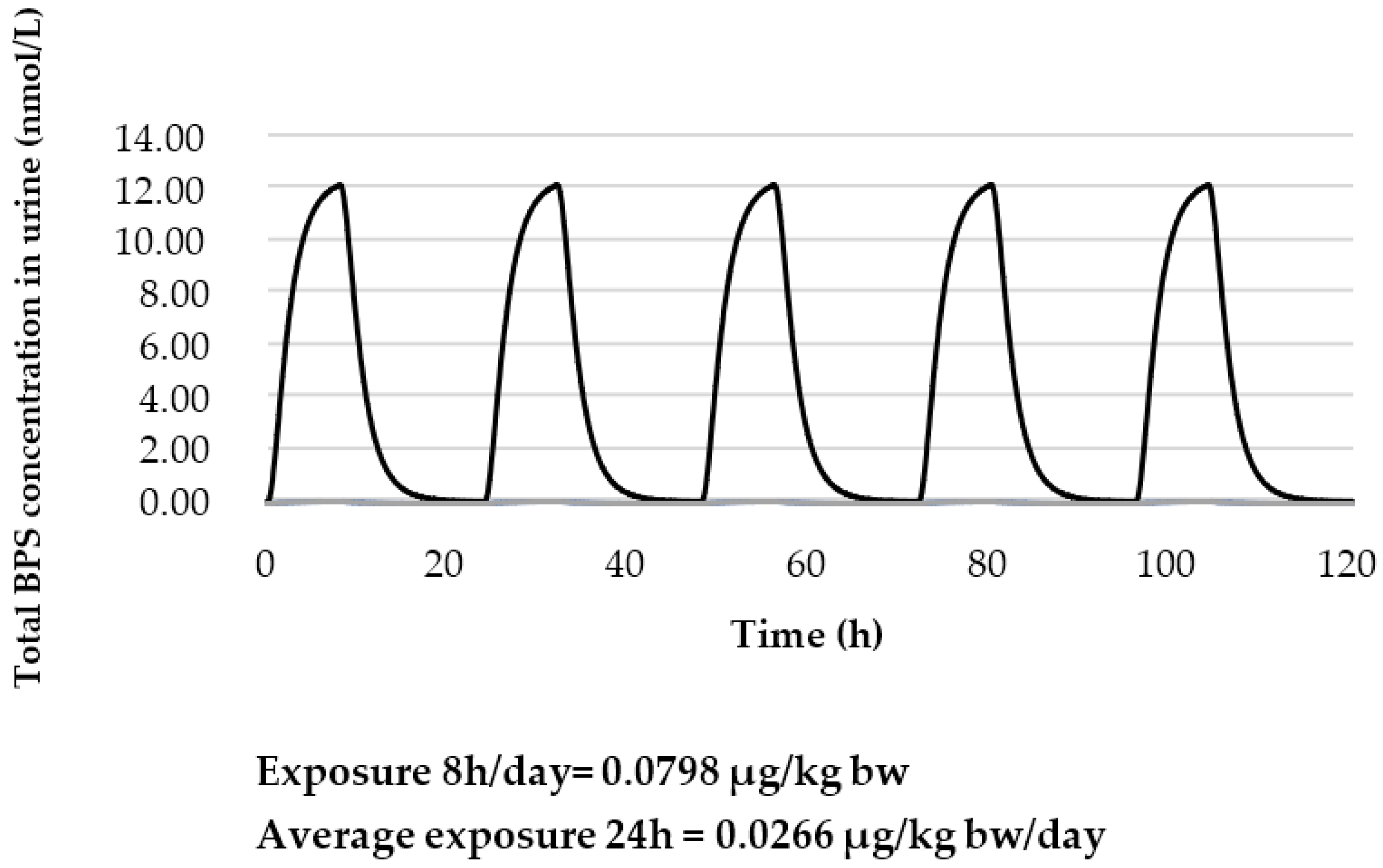
| Key Study | Critical Effect | External Toxicity Reference Value | HBM-GVGenPop | Key Study |
|---|---|---|---|---|
| Tyl et al., 2008 (Two-generation toxicity study in mice) | Increase in relative mean kidney weight in adult males F0 | t-TDI (EFSA, 2015) 4 µg/kg bw/day | 230 µg/L | 135 µg/L |
| Species, Exposure Duration | Critical Endpoint | POD | References |
|---|---|---|---|
| CD-1 Mice, oral exposure, GD 9 to PND 20 | Increased number of terminal end buds in the mammary gland | LOAEL = 2 µg/kg bw/day | Kolla et al., 2018 [63] |
| CD-1 Mice, oral exposure, GD 9 to GD 16 or lactation day 20 | Dose dependent increase in ductal area in the mammary gland. | Kolla et al., 2019 [64] | |
| Rat, oral exposure, GD8/9 to PND20/21 | Neurobehavioral toxicity | Catanese and Vandenberg, 2017 [65] |
| Cohort | Reference | Country | Population | HBM-GVGenPop (µg/L) | P25 (µg/L) | P50 (µg/L) | P75 (µg/L) | P90 (µg/L) | P95 (µg/L) | RCR |
|---|---|---|---|---|---|---|---|---|---|---|
| Esteban | Balicco et al., 2019 [49] | France | Adults (18–74 years old) | 1.0 | 0.14 | 0.31 | 0.80 | 2.24 | 6.33 | 6.3 |
| Adult M (18–74 years old) | 1.0 | 0.15 | 0.38 | 0.88 | 2.44 | 9.71 | 9.7 | |||
| Adult F (18–74 years old) | 1.0 | 0.13 | 0.27 | 0.72 | 2.15 | 5.39 | 5.4 | |||
| Adults (18–29 years old) | 1.0 | 0.16 | 0.44 | 0.99 | 3.38 | 7.13 | 7.1 | |||
| Adults (30–44 years old) | 1.0 | 0.17 | 0.36 | 0.91 | 3.77 | 28.93 | 28.9 | |||
| Adults (45–59 years old) | 1.0 | 0.14 | 0.29 | 0.7 | 1.97 | 3.33 | 3.3 | |||
| Adults (60–74 years old) | 1.0 | 0.10 | 0.22 | 0.66 | 1.59 | 4.11 | 4.1 | |||
| Adults F (18–49 years old) | 1.0 | 0.16 | 0.30 | 0.69 | 2.51 | 6.08 | 6.1 | |||
| Adults F (50 years and older) | 1.0 | 0.11 | 0.23 | 0.78 | 1.98 | 3.49 | 3.5 | |||
| Euromix | Husøy et al., 2019 * [67] | Norway | Adults (24–72 years old) | 1.0 | 0.11 | 0.16 | 0.29 | 0.56 | 0.90 | 0.9 |
| Sakhi et al., 2018 * [68] | Norway | Mothers | 1.0 | <LOQ | <LOQ | 0.26 | 0.44 | 0.59 | 0.6 | |
| Children | 1.0 | <LOD | 0.13 | 0.39 | 0.95 | 1.68 | 1.7 | |||
| TH Pregnant women | Machtinger et al., 2018 [54] | Israel | Pregnant women | 1.0 | <LOD | 0.40 | 0.4 | |||
| SLO-CRP | Tkalec et al., 2021 [55] | Slovenia | Children (6–9 years old) | 1.0 | 0.3 | 0.59 | 0.70 | 0.7 | ||
| Teenagers (11–15 years old) | 1.0 | <LOQ | 1 | 1.80 | 1.8 |
| Reference | Country | Population | HBM-GVGenPop (µg/L) | P50 (µg/L) | P95 (µg/L) | RCR |
|---|---|---|---|---|---|---|
| Ndaw et al., 2018 [69] | France | Professional (cashiers) | 3.0 | 2.53 | 19.9 | 6.6 |
| Professional (controls) | 3.0 | 0.67 | 12.6 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meslin, M.; Beausoleil, C.; Zeman, F.A.; Antignac, J.-P.; Kolossa-Gehring, M.; Rousselle, C.; Apel, P. Human Biomonitoring Guidance Values (HBM-GVs) for Bisphenol S and Assessment of the Risk Due to the Exposure to Bisphenols A and S, in Europe. Toxics 2022, 10, 228. https://doi.org/10.3390/toxics10050228
Meslin M, Beausoleil C, Zeman FA, Antignac J-P, Kolossa-Gehring M, Rousselle C, Apel P. Human Biomonitoring Guidance Values (HBM-GVs) for Bisphenol S and Assessment of the Risk Due to the Exposure to Bisphenols A and S, in Europe. Toxics. 2022; 10(5):228. https://doi.org/10.3390/toxics10050228
Chicago/Turabian StyleMeslin, Matthieu, Claire Beausoleil, Florence Anna Zeman, Jean-Philippe Antignac, Marike Kolossa-Gehring, Christophe Rousselle, and Petra Apel. 2022. "Human Biomonitoring Guidance Values (HBM-GVs) for Bisphenol S and Assessment of the Risk Due to the Exposure to Bisphenols A and S, in Europe" Toxics 10, no. 5: 228. https://doi.org/10.3390/toxics10050228
APA StyleMeslin, M., Beausoleil, C., Zeman, F. A., Antignac, J.-P., Kolossa-Gehring, M., Rousselle, C., & Apel, P. (2022). Human Biomonitoring Guidance Values (HBM-GVs) for Bisphenol S and Assessment of the Risk Due to the Exposure to Bisphenols A and S, in Europe. Toxics, 10(5), 228. https://doi.org/10.3390/toxics10050228







